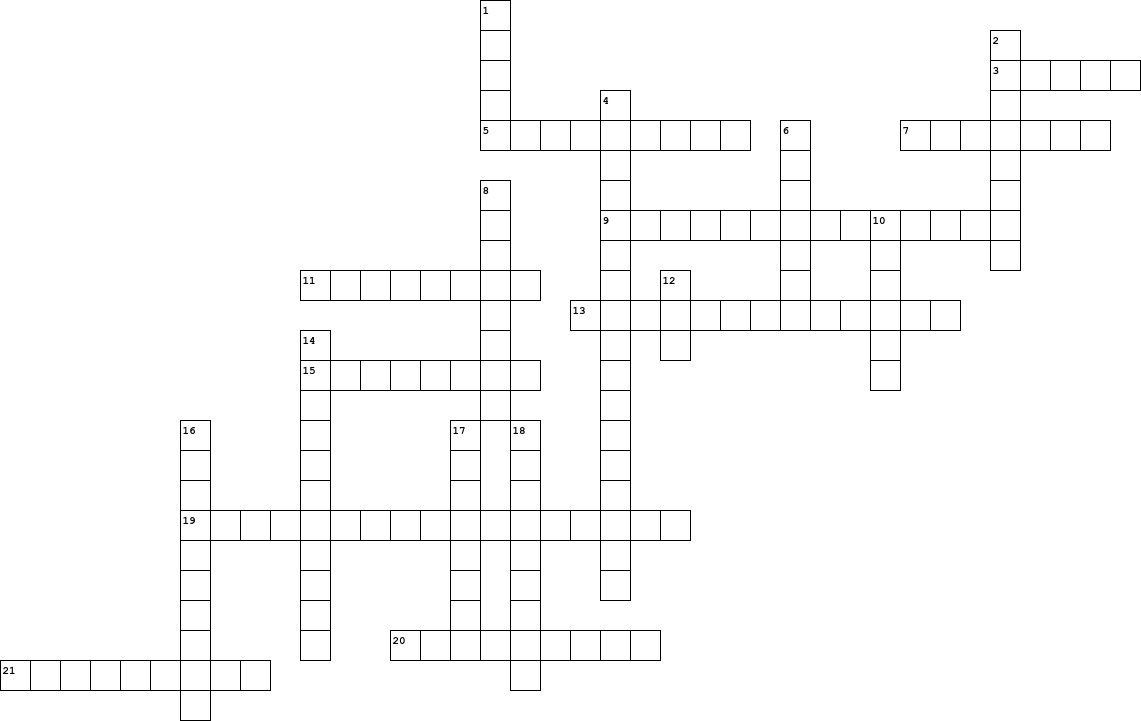
| Down |
| 1. |
Bond between a non-metal and metal atom |
| 2. |
Equation type that show the products and reactants completely balanced. |
| 4. |
Two compounds react and the cation and the anion of the two reactants switch places |
| 6. |
Positive Sub-Atomic particle |
| 8. |
Bond between two atoms that are non-metallic |
| 10. |
A positively charged ion |
| 12. |
+ H2O Product of Combustion (The Equation) (Has a + in it) |
| 14. |
Measures how much energy an atom has |
| 16. |
A method to help find the chemical formula of Ionic compounds |
| 17. |
Equation type that simply shows the products and reactants. Unbalanced |
| 18. |
Negative Sub-Atomic particle |